A Novel Platinum Nanocluster for Improved Oxygen Reduction Reaction in Fuel Cells
Scientists have elucidated the reason for the new catalyst’s high activity, which is 2.1 times higher than commercial platinum nanoparticle-based catalysts
Hydrogen, derived from polymer electrolyte fuel cells (PEFCs), is an excellent source of clean energy. However, PEFCs require platinum (Pt), which is a limited resource. Some studies have shown that Pt nanoclusters (NCs) have higher activity than conventionally used Pt nanoparticles, however the origin of their higher activity is unclear. Now, researchers have synthesized a novel Pt NC catalyst with unprecedented activity and identified the reason for its high performance.
The twin issues of climate change and the shortage of fossil fuels are the new cornerstones challenges of energy research. Polymer electrolyte fuel cells (PEFCs), which produce the clean fuel hydrogen, are one of the most promising options to tackle both these challenges. However, PEFCs are expensive to make and operate, primarily because of the large amount of platinum (Pt) that they require. Moreover, the amount of Pt in the Earth’s crust is limited, which means that to make PEFCs truly sustainable, it is imperative to reduce the amount of Pt that they use. Presently, PEFCs use cathodes (the positive electrode) made with Pt nanoparticles (NPs) that are supported on carbon black (Pt NPs/CB). However, recent research has indicated that Pt nanoclusters (Pt NCs) have higher oxygen reduction reaction (ORR) activity than Pt NPs, i.e., they have higher performance. Thus far, the reason for Pt NCs high ORR activity has been unclear.
Recently, a research team led by Professor Yuichi Negishi from Tokyo University of Science (TUS) have developed a novel Pt NC that exhibits 2.1 times higher ORR activity than commercial Pt NPs and elucidated the origin of its high activity. “In our study, we focused on Pt NCs derived from a Pt, carbon carboxylate (CO), and triphenylphosphine (PPh3) base i.e., [Pt17(CO)12(PPh3)8]z (where z = 1+ or 2+). We recently showed that these Pt NCs, unlike others, are stable in air. We then performed density functional theory (DFT) calculations to reveal the reason for its remarkable activity,” says Prof. Negishi. The research team also included Junior Associate Professor Tokuhisa Kawawaki from Tokyo University of Science, Associate Professor Kenji Iida from Hokkaido University, Professor Toshihiko Yokoyama from Institute for Molecular Science, Japan, and Professor Gregory F. Metha from the University of Adelaide, Australia. The study has been published in the journal Nanoscale on 24 March 2023.
The researchers prepared the Pt NCs by the adsorption of [Pt17(CO)12(PPh3)8]z onto carbon black, followed by a calcination reaction. They then compared its performance to conventional Pt NPs/CB using a technique called linear sweep voltammetry. They found that the novel Pt NCs had higher performance than the Pt NPs/CB. Notably, at 0.9 volts, the Pt NCs had 2.1 times higher activity than the Pt NPs/CB. They also found that increasing Pt loading in the electrode leads to an increase in its mass activity, and that the PT NCs had higher durability than the commercial PT NPs/CB.
Next, to elucidate the origins of its high activity, they performed DFT calculations. “Our calculations suggest that the high ORR activity of the novel Pt NCs is due to the surface Pt atoms, which have an electronic structure that is suitable for the progress of ORR,” reveals Prof. Negishi.
These findings can serve as a guideline for the design of future high-activity, high-performance Pt catalysts for use in PEFCs, which will take us one step further towards mitigating climate change and the fossil fuel crisis.
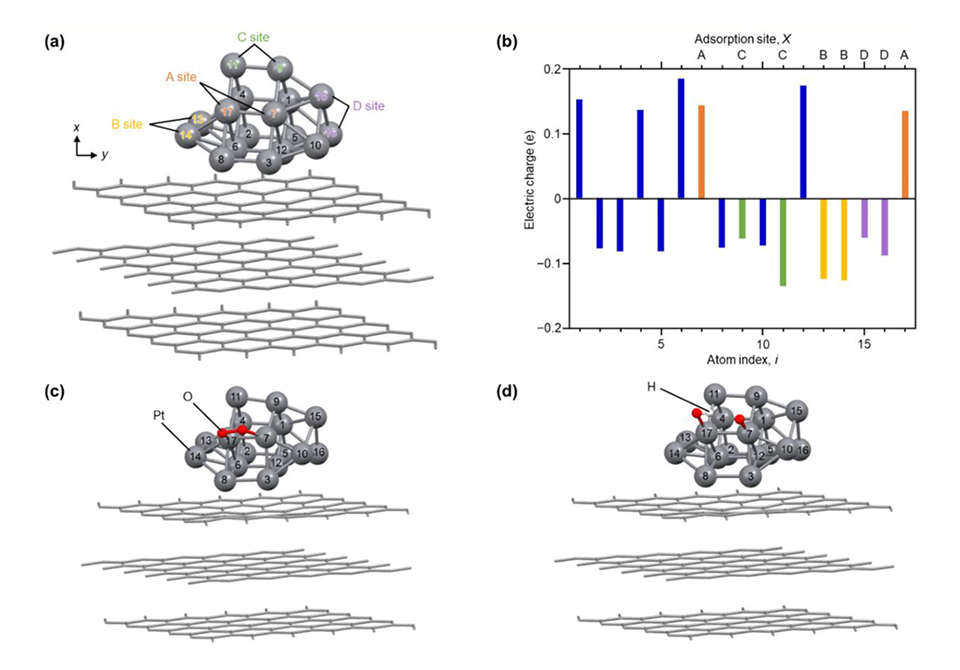
Image title: DFT calculation results of optimized Pt atoms in Pt17 on graphite, describing the interaction of oxygen with each Pt atom, shown with atom index.
Image caption: The origin of high ORR activity of (a) Pt17/CB can be attributed to: (b) variable electric charges of each Pt atom on the nanocluster, (c) represented intermediate structure optimized for O2/Pt17/graphite and (d) intermediate structure optimized for (O + OH)/Pt17/graphite.
Image credit: Yuichi Negishi from TUS, Japan
License type: CC BY-NC 3.0










